How to Compute Molecular Weight
The molar mass of a compound (sometimes imprecisely referred to as molecular weight) is the weight of one mole of that substance expressed in grams per mole. One mole of any quantity is equivalent to 6.0221415 x 1023 units. For example, the molar mass of water (H2O) is 18.01528 g/mol, which means that the weight of 6.0221415 x 1023 water molecules is 18.01528 grams.
To calculate the molar mass of a compound, you need to know its chemical formula and the atomic masses of each element that comprises the compound. The molar mass is simply the sum of the atomic weights of each element. For example, one water molecule is made of two hydrogen atoms and one oxygen atom. Since the atomic weight of hydrogen is 1.00794 and the atomic weight of oxygen is 15.9994, this means that the molar mass of water is 1.00794 + 1.00794 + 15.9994 = 18.01528.
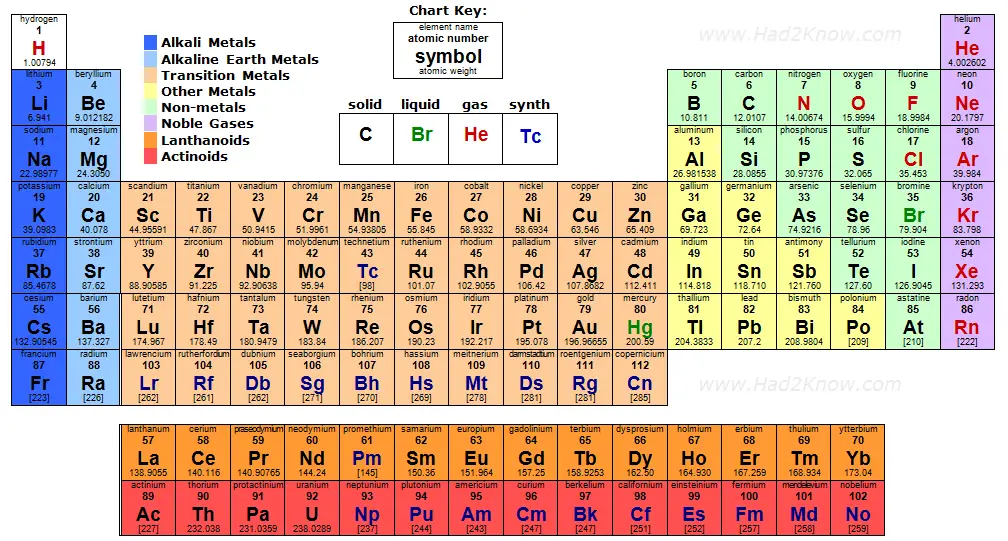
You can easily calculate the molar mass of various compounds with the aid of a periodic table of elements, such as the one linked below. You can also use the convenient molar mass calculator on the left.
To enter a chemical formula into the molar mass calculator, use the IUPAC abbreviations for the elements. You can also use parentheses to denote chemical groups. For example, trimethyl borate can written as B(OCH3)3, BO3(CH3)3, (CH3)3BO3, or C3H9BO3.
Click the image thumbnail to see the full-size periodic table of elements in a new tab or window.
Atomic masses in brackets indicate the atomic mass of the most stable isotope known.
© Had2Know 2010