Density of Ice and Liquid Water Calculator
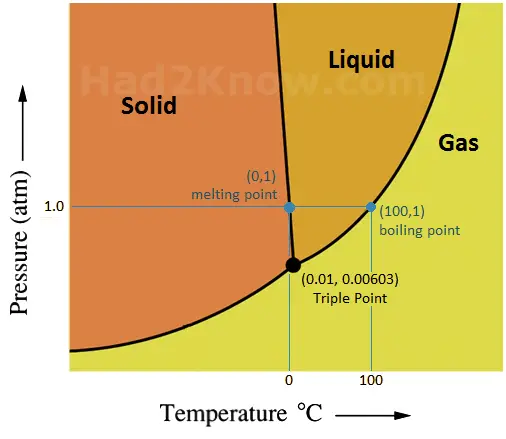
The state of water--solid, liquid, or gas--depends on the temperature and pressure, and the density of water depends on its state and temperature. At standard atmospheric pressure (1 atm), liquid water becomes a solid at 0°C and becomes vapor at 100°C. Under higher pressure water can exists as a super-cooled liquid below 0°C, or as a super-heated liquid above 100°C. See phase diagram below.
When water transitions from a liquid to a solid (freezes), its density decreases by about 9%, which is unusual among chemical compounds. This is the reason why ice floats in liquid water. When water is in the ice state, it's density starts to increase slightly as the temperature falls further below zero, though it never becomes as dense as liquid water. At 0°C, ice has a density of 0.9162 g/mL, and at -100°C its density is 0.9257 g/mL.
When water is in its liquid state, its density decreases as the temperature increases. At 0°C, liquid water has a density of 0.9998425 g/mL, at 100°C liquid water's density is 0.9583665 g/mL. The maximum density of liquid water does not occur at 0°C, but at 3.98°C. As water is heated from 0°C to 3.98°C the density slightly increases before it begins to decrease.
You can use the calculator on the left or the tables below to estimate the density of solid and liquid water at various temperatures.
Phase Diagram of Water
Density Tables
| Liquid H₂O | |
| Temperature °C | Density g/mL |
| -5 | 0.999259 |
| 0 | 0.9998425 |
| 5 | 0.9999668 |
| 10 | 0.9997026 |
| 15 | 0.9991026 |
| 20 | 0.9982071 |
| 25 | 0.9970479 |
| 30 | 0.9956502 |
| 35 | 0.9940349 |
| 40 | 0.9922187 |
| 45 | 0.9902162 |
| 50 | 0.9880393 |
| 55 | 0.9856982 |
| 60 | 0.9832018 |
| 65 | 0.9805578 |
| 70 | 0.9777726 |
| 75 | 0.9748519 |
| 80 | 0.9718007 |
| 85 | 0.9686232 |
| 90 | 0.9653230 |
| 95 | 0.9619033 |
| 100 | 0.9583665 |
| 105 | 0.954733 |
| Solid H₂O (Ice) | |
| Temperature °C | Density g/mL |
| -100 | 0.9257 |
| -90 | 0.9249 |
| -80 | 0.9241 |
| -70 | 0.9233 |
| -60 | 0.9224 |
| -50 | 0.9216 |
| -40 | 0.9208 |
| -30 | 0.9200 |
| -20 | 0.9194 |
| -10 | 0.9189 |
| 0 | 0.9162 |
© Had2Know 2010
